Introduction
Calorimetry is useful tool for monitoring cell cultures in situ. Cellular growth is accompanied by production of heat, which is proportional to the rate of substrate consumption and waste formation. Using the titration mode of Micro Reaction calorimeter (μRCTM) from THT, the direct effect of inhibitor or substrate addition to a culture can be measured.
This application note presents data on bacterial growth in semi-skimmed milk, inoculated with yoghurts, as monitored in the μRCTM.
Experimental
The μRCTM was set to 35℃, 1.25 mL of smei-skimmed milk inoculated with 25 μL yoghurt was placed in the sample vial. An equal volume of water water was placed th the sample vial. The isothermal data collection mode was used to record the heat produced over a 19-hour period. Further experiments were run with the same amounts of milk and yoghurt with the following additives being added by injection:
- 3% hydrogen peroxid solution
- a 0.6 mM solution of the bacteriostatic oxytetracycline
- water
The heat versus time response was rerorded for 3 hours before and 16 hours after the injection.
Results
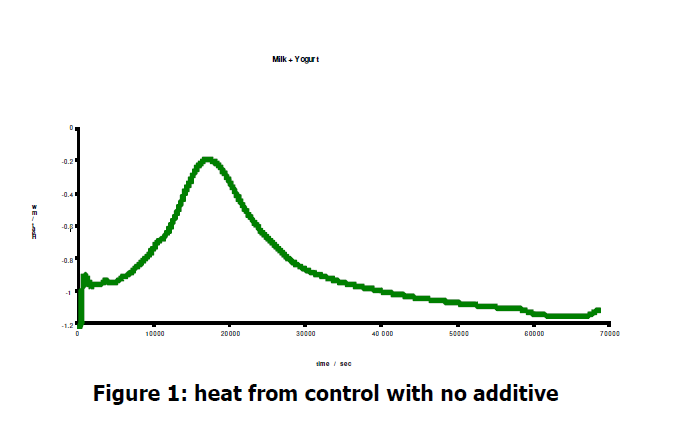
The heat data from the experiments is illustrated in Figures 1-4. Figure 1 show the contcol data, with the mixture of milk and yoghurt being left inside the μRCTM for 19 hours.
The sample follows a typical Gaussian growth curve with a maximum heat output of 0.9 mW. The sample was contained in a sealed HPLC vial; as the concentration of toxins increases sna the oxygen is used up, the bacteria begin to die. This is indicated by the decreases in heat signal.
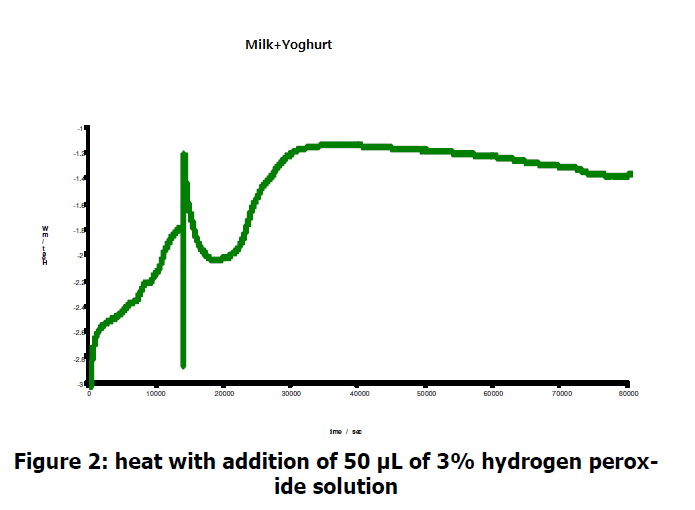
Hydrogen peroxide is known to kill bacteria. Upon addition of peroide, the heat output drops significantly before increasing again after roughly 3 hours.
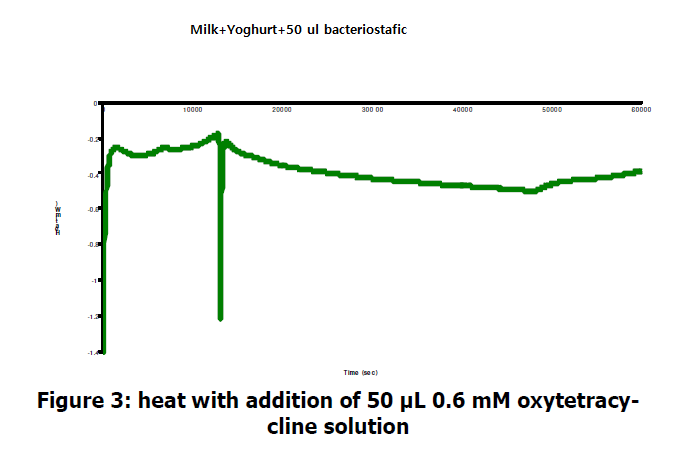
Oxyteteracyline is a bacteriostatic( i.e. inhibitor of bacterial growth) and is thought to exert its antimicrobial effect by the prevention of the protein synthesis. Figure 3 overleaf shows that adding 50 μL 0.6 mM Oxytetracyline instanly stops any further increase in that production, indicating no further bacterial growth is taking place.
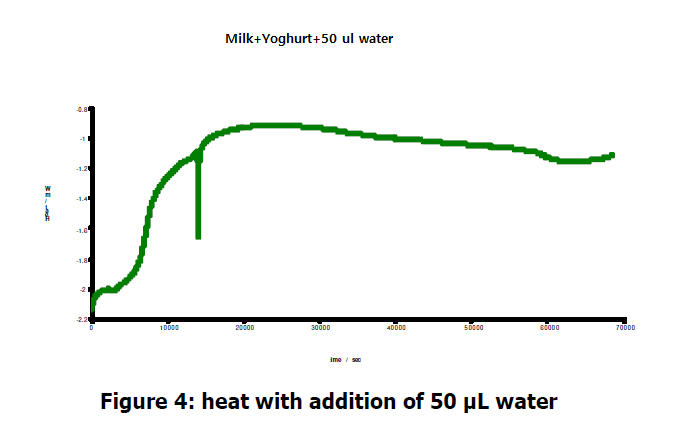
As a further control experiment, 50 μL of water was added to another bacterial culture. The that output continued to rise, showing that the bacterial growth was unaffected.
Discusstion and conclusions
An overlay of the heat data in Figure 5 allow for a direct comparision between the bacterial growth rates under different conditions.
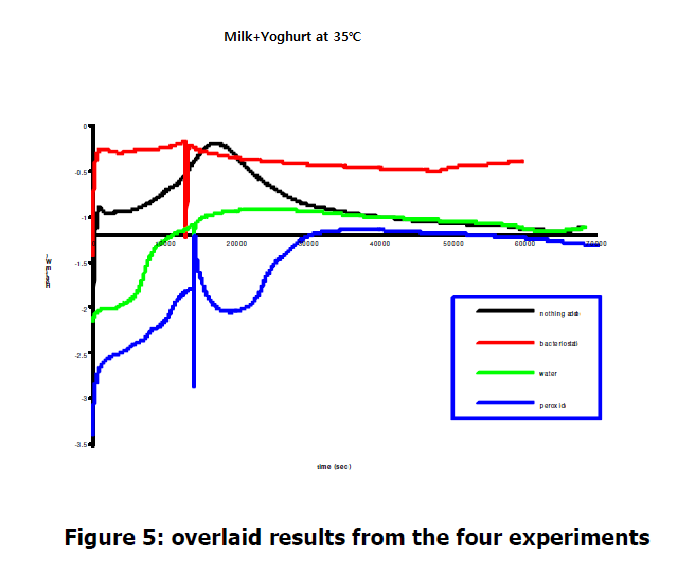
The ablilty of the μRCTM to the derect the heat associated with bacterial growth demostrates the sitability of the instrument to study microorgaism metabolism and the level of resistance to different antibiotics. Its repid response time and ease of use allow anumber of quantitative studies to be carried out with relatively little user input.
The THT Micro Reaction Calorimeter (µRC™) has a wide range of applications in the chemical, pharmaceutical and related fields. This brochure shows how using a microcalorimeter can help chemists and engineers in everyday tasks.
Some of the applications detailed within this brochure include:
- Reaction Kinetics
- Process Development
- Scanning Calorimetry
- Thermal Stability
- Heat Capacity Measurement
- Adhesive Curing Reactions
- Waste Management
- End Point Determination
- Hazard Analysis
All chemical, physical and biological reactions are accompanied by heat change. These reactions, though sometimes subtle, can be measured using calorimetry. This powerful analysis method works without modification of the sample or process. The THT Micro Reaction Calorimeter is based on power compensation technology making it faster in both signal response and temperature variation. Designed for maximum flexibility, the µRC has the capability to measure both kinetic and thermodynamic parameters in both rapid and slow reactions and in solids, liquids or gases. Measurements made by calorimetry are non-destructive and non-invasive making it valuable for initial analyses and for systems where other techniques fail. With the µRC there is minimal sample preparation and no limitation on the physical state of the material. Systems can be studied under ambient or modified environmental conditions.
Specification
- Baseline Noise: From 5μW
- Dynamic Range: 5μW to 600mW
- Temperature Range: -5°C to 170°C
- Standard Modes of Operation: Isothermal/Titration/ Scanning
- Optional Modes: Pressure - pressurise cell up to 10bar
uRC를 이용한 음식물의 부패와 미생물 성장관찰
(Food Spoilage and Microbial Growth) - Scanning Rate: Up to 2°C/min
- Isothermal Stability: +/- 0.0001°C over extended time period
- Cell Volume: 1.5 ml
- Cell Type: Removable glass vial
- Injection Volume: 1 to 250 μl
- Temperature Control: Peltier based (no external cooling)
- Stirring Speed: 0 - 400 rpm
- Measurement Principle: Power compensation
- Connection to PC: via USB cable
- Footprint (width x depth x height): 19 x 31 x 35 cm
- Certain other specifications may be possible by discussion
하기
