Introduction
Melting point standards can be used to check the temperature calibration of instrument. Some compounds also have a known enthalpy of fusion which can be compared to a literature value as a further test of heat measurement accuracy. The Micro Reaction calorimeter (μRCTM) from THT has a scanning function which allows the user to ramp the temperature from -10 to 160℃ at a specified rate. This method can be used to determine the melting point of an unknown compound (see Figure 3 overleaf). Scanning can also be used to measure the enthalpy of fusion based on the absorbed heat, and gain qualitative information on purity based on the shape of peak.
Experimental
For a typical scan test, 19.4 mg indium was weighed into a sample vial and secured with a heat resistant lid, was placed in the reference cell. A scan test was initiated starting a 100℃and ending at 160℃, with a ramp rate of 1℃/min.
Result
Figure 1 shows the temperature data from the experiment. The temperature is held constant at 100℃ than increases steadily up to 160℃.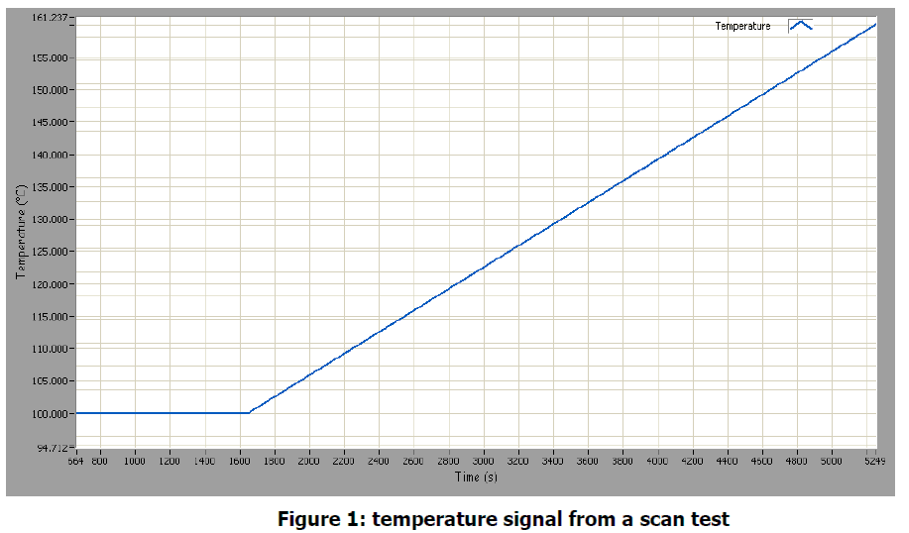
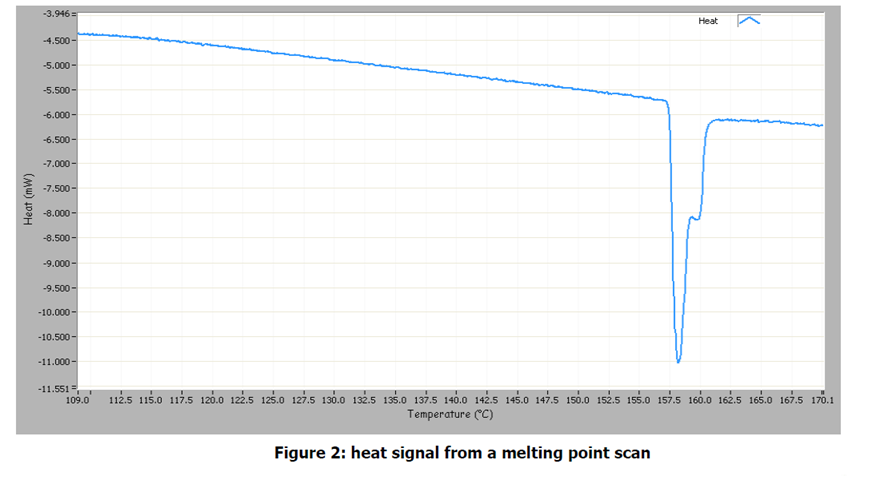
Figure 2 shows the power data from the experiment. The baseline shows a linear decline as the temperature decreases. The endothermic peak corresponds to heat absorption by the indium as it melts. The integrated area of the peak in Figure 2 is 0.544 J. This can be divided by the mass of indium in the sample to give the enthalpy of fusion.
0.544 J/0.0194 g=28.0 J/g
This compares well to the literature value of 28.7 J/g1)
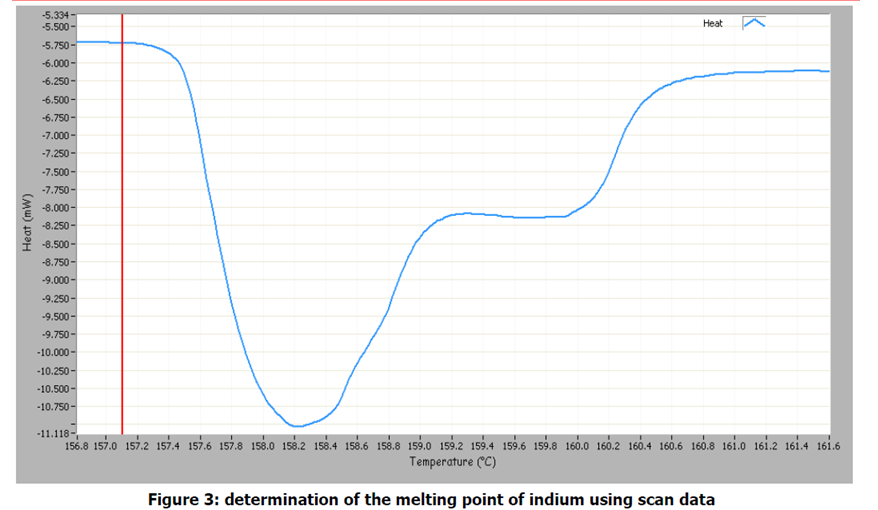
The μRC Analysis software allow the heat signal to be viewed as a function of temperature or time. Figure 3 shows the heat/temperature graph being used to determine the melting point of indium. The melting point is defined as the temperature at which melting begins, which in terms of heat is the very start of the peak. This corresponds to a temperature of 157.1℃. The literature value is 156.6℃2).
Discussion and conclusions
It has been demonstrated that a simple, 60-minute scan test on the μRCTM can be used to obtain quantitative melting data from a small sample. The method is not limited to pure substances, and should be accurate with samples as small as 10 mg.
The sharp drop in the heat signal in figure 3 at the beginning of the melting process suggest a compound of high purity. A more gradual decline, or a decrease in the melting point, would indicate impurities in the sample. It should be noted that not all melting point standards are suitable for measurement of the enthalpy of fusion. Some solids may partially sublime, and the nature of the sealed vials in the μRCTM make the instrument unsuitable for determining enthalpies of sublimation. Other melting point standards which have been used for temperature calibration testing include diphenyl ketone, benzoic acid, naphthalene and phenyl salicylate.
References
1)D. G. Archer, S. Rudtsch; J. chem. Eng. Data, 2003, 48(5), pp. 1157-1163
2)http://www.chemicalelements.com/elements/in.html
The THT Micro Reaction Calorimeter (µRC™) has a wide range of applications in the chemical, pharmaceutical and related fields. This brochure shows how using a microcalorimeter can help chemists and engineers in everyday tasks.
Some of the applications detailed within this brochure include:
- Reaction Kinetics
- Process Development
- Scanning Calorimetry
- Thermal Stability
- Heat Capacity Measurement
- Adhesive Curing Reactions
- Waste Management
- End Point Determination
- Hazard Analysis
All chemical, physical and biological reactions are accompanied by heat change. These reactions, though sometimes subtle, can be measured using calorimetry. This powerful analysis method works without modification of the sample or process. The THT Micro Reaction Calorimeter is based on power compensation technology making it faster in both signal response and temperature variation. Designed for maximum flexibility, the µRC has the capability to measure both kinetic and thermodynamic parameters in both rapid and slow reactions and in solids, liquids or gases. Measurements made by calorimetry are non-destructive and non-invasive making it valuable for initial analyses and for systems where other techniques fail. With the µRC there is minimal sample preparation and no limitation on the physical state of the material. Systems can be studied under ambient or modified environmental conditions.
Specification
- Baseline Noise: From 5μW
- Dynamic Range: 5μW to 600mW
- Temperature Range: -5°C to 170°C
- Standard Modes of Operation: Isothermal/Titration/ Scanning
- Optional Modes: Pressure - pressurise cell up to 10bar
μRC에 의한 melting temperature 측정 - Scanning Rate: Up to 2°C/min
- Isothermal Stability: +/- 0.0001°C over extended time period
- Cell Volume: 1.5 ml
- Cell Type: Removable glass vial
- Injection Volume: 1 to 250 μl
- Temperature Control: Peltier based (no external cooling)
- Stirring Speed: 0 - 400 rpm
- Measurement Principle: Power compensation
- Connection to PC: via USB cable
- Footprint (width x depth x height): 19 x 31 x 35 cm
- Certain other specifications may be possible by discussion
하기
