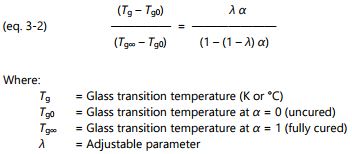
Stages of thermoset cure
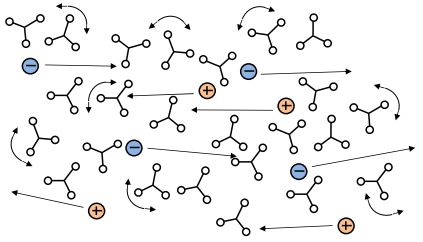
A-Stage thermoset
Thermosets are an important class of materials used for adhesives, coatings and composites. They include epoxies, (poly)urethanes, acrylics, phenolics, vinyl esters, silicones and many other compounds. Uncured thermosets, or A-stage materials, are composed of small molecules called monomers, as shown in Figure 3-1.
B-Stage thermoset (partial curing, before gel point)
With the application of a catalyst, hardener, or energy such as heat or light, these monomers react and bond to one another to form longer and longer chains called polymers. Once curing begins, but while still fluid, the thermoset is a B-Stage material, represented in Figure 3-2. During this period the number of molecules decreases while their molecular weight

increases. The thermoset’s viscosity also increases, as does its resistance to the flow of mobile ions in an electric field. Dipoles in the polymer can rotate in response to an oscillating electric field, and this ability to rotate also decreases as cure advances.
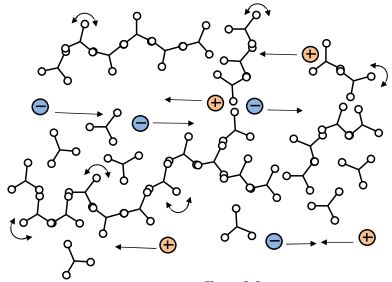
Gel point (start of infinite network)
Through the process of crosslinking, which is the formation of bonds that link one polymer chain to another, an extended branching network appears as shown in Figure 3-3. Crosslinks restrict the movement of polymer chains and the thermoset’s viscosity increases rapidly.
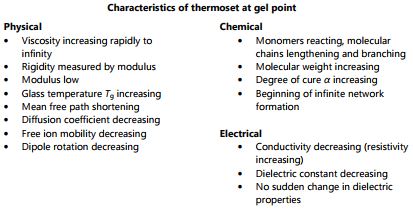
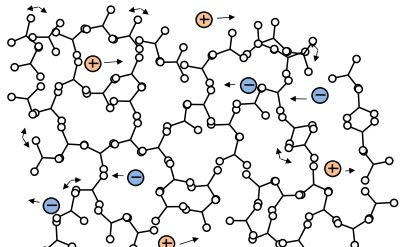
C-Stage resin (end of cure)
At some point the network essentially becomes a single molecule of infinite molecular weight, and the beginning of infinite network formation is called gelation or the gel point—the material changes from a viscous liquid that can flow to a gel or rubber that cannot.
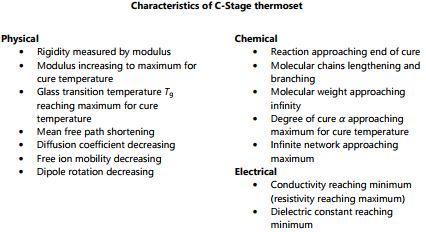
Note that gelation is a mechanical condition that does not cause a corresponding change in electrical properties. After gelation the thermoset hardens into a solid. Upon full cure the thermoset is a C-Stage material as shown in Figure 3-4. At constant temperature, as a thermoset cures from A-Stage to B-Stage to C-Stage, both free ion mobility and the amount of dipole rotation decrease continuously. Ion mobility and dipole rotation vary with temperature as well as cure state, however, and their behavior is more complex if these factors change at the same time. The electrical quantities of resistivity and permittivity depend on free ion mobility and dipole rotation, respectively. As a result, these dielectric properties correlate with viscosity before gelation, and with rigidity or modulus after gelation.
Degree of cure and glass transition temperature
The degree of cure α is a measure of the amount of reaction for the thermoset. Each bond releases a fixed amount of heat, and the degree of cure is defined as:
The degree of cure also correlates with crosslink density ; α therefore is useful for indicating physical state. A material undergoes a glass transition when it changes from a glassy and relatively brittle solid to one that is rubbery and relatively soft. Above the glass transition temperature T g (actually a range of temperature) a polymer is rubbery because sufficient thermal energy is available to increase the flexibility of crosslinks. Below T g the polymer vitrifies and is rigid. Like degree of cure, glass transition temperature increases with crosslink density, increases as cure progresses and is a measure of cure state. The DiBenedetto model, below, is often used to relate degree of cure to glass transition temperature.
The relationships among degree of cure, glass transition temperature and electrical properties of the thermoset are the basis for dielectric cure monitoring, which uses electrical measurements to measure cure.
Reference :
Lambient TechnologiesTM Application Note 3.03—Basics of Thermoset Cure, All Page
상세한 사항은 아래 첨부된 자료를 참고 부탁 드립니다.
검색 결과 15건
- 분류 : 유전율측정기