Retardation of cement: sucrose and xylitol
Application Note AN-C09
Instruments to which this note applies: Calmetrix I-Cal Flex isothermal calorimeter
Target use: Setting time estimation, materials selection, mix design optimization, admixture formulation, quality control in cement manufacturing, dry mortar
Summary
The retarding effect of sucrose and xylitol on the hydration of a Portland-limestone cement was investigated by isothermal calorimetry. It was found that the retarding effect of both added substances was proportional to their concentrations, and that sucrose was about 25 times more retarding than xylitol.
Introduction
등온 시멘트 열량 측정법의 중요하고 다소 쉬운 작업 중 하나는 응결지연 특성을 정량화하는 것이다. 대부분의 경우 강도를 주는 알라이트 수화 피크 (alite hydration peak)의 onset을 지연시킴으로써 많은 물질이 시멘트 수화의 응결을 지연시키는 것으로 잘 알려져있다.
많은 물질에 의해 수화가 지연되며, 아연과 납과 같은 중금속 [1], 붕소 화합물 (boron compound) [2], 링고 설포네이트 가소제 (lingosulfonate plasticizer) [3]가 포함된다.
매우 효과적이고 잘 알려진 시멘트 수화 응결 지연제는 표면 흡착 (surface adsorption) 및 growth site poisoning에 의해 작용하는 보통 우리가 알고 있는 설탕 (수크로스)이다 [3].
The retarding effect is strong; a typical statement on the internet is “Tests have shown that 0.05 percent (by weight of cement) of a refined granulated sugar included in a normal mix will retard setting by approximately 4 hours” [4] (however, it is advisable to instead use commercial retarders for a more consistent result). The present application note answers the following question: is the sweetener xylitol also a cement hydration retarder?
Materials and method
A commercial Portland-limestone cement was used with tap water and high purity sucrose and xylitol. The samples were made by weighing 2 g of cement into 20 mL plastic vials and then adding about 1 g of water with sucrose or xylitol and mixing for 30 s on a vortex mixer at high speed. Measurements were made in six calorimeters in an I-Cal Flex M isothermal calorimeter.
Results and discussion
The results of our measurements are shown in Fig. 1, with the sucrose additions to the left and the xylitol additions to the right. It is seen that there is a slight retardation with sucrose – the hydration curves are shifted to the right – but that the effect is small or negligible for xylitol. Note that the concentrations of added substances are low.
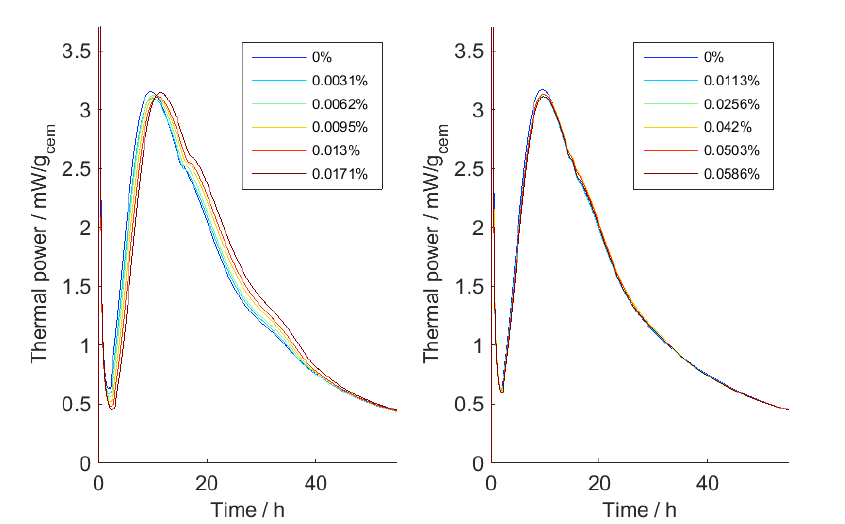
To quantify the kinetics of the cement hydration we used the time of the maximal thermal power. In Fig. 2 these times are plotted for the curves shown in Fig. 1 and it is seen that it is possible to quantify the retardation and that it is quasi-linear in the concentration ranges used.
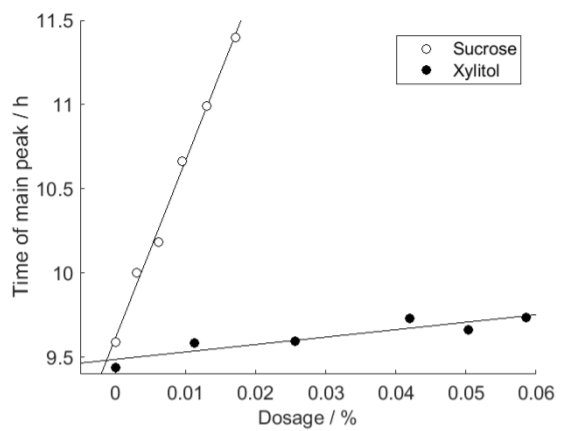
The slopes of the lines in Fig. 2 show how much the hydration peak shifts to the right per mass percent addition of sucrose or xylitol (relative to the mass of cement). The slopes are about 100 and 4 for sucrose and xylitol, quantitatively showing that sucrose is about 25 times more retarding than xylitol.
The precision of the measurements were good even if the measurements were rather easy to make. The standard deviation around the regression lines was about 3 min and 30 min for sucrose and xylitol, respectively. This shows that it is possible to qualitatively evaluate retardation from measurements with isothermal calorimetry.
Conclusion
Xylitol is much less active as a retarder than sucrose. Isothermal calorimetry is an excellent method to quantify retardation, and the effect of even small additions of retarders can be quantitatively evaluated.
Supplementary information
The different concentrations of sucrose or xylitol were made by first adding a certain number of drops of an aqueous sucrose or xylitol solution on the cement and then adding water to get 1 g. Each of the two measurement series thus have samples with 0, 1, 2, 3, 4 and 5 drops of sucrose of xylitol solution. This was made to avoiding making more than one sucrose and one xylitol solutions. A possible drawback with this method of making samples with different concentrations of the added substances, is that the added substances first come in contact with the cement at a higher concentration than their final concentration. Above we used the time of the main peak as an indication of the kinetics of the hydration. We could also have used other indices for this, for example, the onset of the main hydration peak, the time to reach (for example) 1 mW/gcemor the timing of the sufate depletion peak, and would at least in the present example got about the same results. From a chemical point of view it is not surprising that xylitol is less active as a retarder than sucrose. Although xylitol is a strong sweetener, it is not a sugar, but a sugar alcohol, and has different chemical properties than sucrose. This has been studied in some detail by Zhang et al. [5] who found that different sugar alcohols had different retarding effects on cement hydration, and that this could be related to their chemical structures. A more general discussion of how admixtures influence cement hydration is given by Cheung et al. [6].
References
[1] C. Weeks, R.J. Hand and J.H. Sharp, Cement & Concrete Composites, 30, 970-978 (2008).
[2] J. Kratochvíl, T. Opravil and P. Diviš, Adv. Mater. Res., 1000, 16-19 (2014).
[3] M. Bishop and A.R. Barron, Ind. Eng. Chem. Res., 45, 7042-7049 (2006).
[4] http://www.concreteconstruction.net/how-to/sugar-as-aretarder_o.
[5] L. Zhang, L.J.J. Catalan, R.J. Balec, A.C. Larsen, H.H. Esmaeili and S.D. Kinrade, J. Am. Ceram. Soc., 93, 279-287 (2010).
[6] J. Cheung, A. Jeknavorian, L. Roberts and D.A. Silva, Cement Concrete Res., 41, 1289-1309 (2011).
