
Instruments to which this note applies: I-Cal Flex, Biocal 2000, Biocal 4000
Target use: Research and Quality Control related to food spoilage and effectiveness of preservation methods.
Introduction
부패는 외형이나 사람의 소비에 대한 적합성으로 식품의 품질을 저하시키는 모든 유형의 과정을 말합니다. 부패는 종종 효소 활성도, 산화 또는 미생물 생장 (박테리아, 곰팡이, 효모)를 통해 발생됩니다. 부패는 주로 외부 변화와 관련이 있지만 눈에 띄는 징후가 나타나기 오래 전에 시작될 수 있습니다. 등온 열량계는 열이 방출되는 것을 지속적으로 측정하므로 부패 가 실질적으로 시작되는 시간을 알아 내는 데 사용할 수 있습니다. 샘플로부터 발생되는 열을 전력 (power)으로 측정함으로써, 반응의 동역학 (kinetics of reaction, 반응속도), 즉 부패가 얼마나 빠르게 진행되는 지 결정할 수 있습니다. 특별한 technician의 도움없이도 측정이 가능하며, 버튼 클릭만으로도 편리하게 결과를 검색할 수 있으며 실험이 완료되는 동안 또는 완료된 후 언제든지 평가와 해석이 가능합니다.
Isothermal calorimeters are a convenient and cost effective tool to quickly assess how spoilage develops in different food items, and which preserving methods work best, simply by comparing their isothermal calorimetry curves. Using a large sample cell calorimeter such as the Calmetrix Biocal, with 125 ml sample vials, increases the range of applications by making it possible to study food items such as pieces of meat or cheese and whole small fruit.
This Application Note shows the thermal activity in two samples of commercial lightly pasteurized fruit drink made from blueberries, containing blueberries, black currents, grapes and sugar.
Test Protocol
Both sample vials were loaded with about 100 g of fresh blueberry and black current juice in a controlled temperature at 23 °C, but in one of the samples a small amount of a contaminant was added.
Results and Interpretation
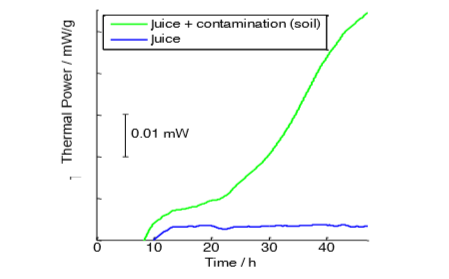
Both samples show relatively low thermal powers, but the thermal power of the sample with a contaminant increases in an exponential fashion, while the sample without contaminant shows a constant thermal power over an extended period of time.
In this experiment the 100 g samples were taken from a refrigerator and then loaded into the calorimeter, which is why the initial part of the curve shows negative thermal power as the temperature in the sample stabilizes to the set temperature inside the calorimeter. Alternatively, it would be possible to pre-heat the samples in a heating block. However, as shown in this note, even a measurement protocol without pre-heating yields perfectly relevant results that show the significant influence of the contaminant on the shelf life of the product.
Calorimetry measurements such as these, on juices produced with different pasteurization procedures, preservatives and their concentrations, pH or other production variables can be used to easily identify the best method to extent shelf life.
Conclusion
Isothermal calorimeters such as Biocal 2000 and Biocal 4000 are an effective and easy-to-use tool for shelf life studies. The method described in this Application Note can be used for all types of liquid or solid foodstuffs and for many different processes. Microbiological spoilage, as in the present measurement, produces relatively high thermal powers and is therefore easy to study with isothermal calorimetry.
References
The following references discuss shelf life studies using isothermal calorimetry in food science.
- Riva, M., D. Fessas, and A. Schiraldi, Isothermal calorimetry approach to evaluate shelf life of foods. Thermochim. Acta, 370 (2001) 73-81.
- Alklint, C., L. Wadsö, and I. Sjöholm, Accelerated storage and isothermal microcalorimetry as methods of predicting carrot juice shelf-life. J. Sci. Food Agric., 85 (2005) 281-285.
- Wadsö, L. and F. Gómez Galindo, Isothermal calorimetry for biological applications in food science and technology. Food Control, 20 (2009) 956-961.
