Dependence of ion viscosity on temperature and cure state
Dependence of ion viscosity on temperature and cure state When temperature is constant, it is easy to understand how dielectric measurements indicate cure state—only the degree of cure affects the ion viscosity of a thermoset or composite. However, ion viscosity actually depends on both degree of cure and temperature, and accounting for temperature is necessary to correctly interpret non-isothermal tests. Two rules can sum up the behavior of ion viscosity:
1. At constant degree of cure, increasing (or decreasing) temperature decreases (or increases) ion viscosity
2. At constant temperature, increasing (or decreasing) degree of cure increases (or decreases) ion viscosity
Because these rules have opposite effects, the net result depends on which dominates at a particular moment. Figure 21-1 plots data from the cure of an epoxy-fiberglass prepreg.
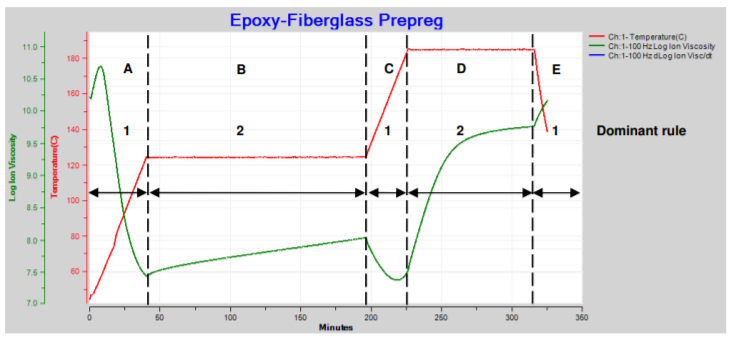
Epoxy-fiberglass prepreg cure with ramps and holds
Here ion viscosity changes as temperature goes through two ramp and hold steps then a final cooling:
A. Temperature increases but is too low to initiate significant reaction, so degree of cure is essentially constant and ion viscosity decreases (Rule 1 dominates). B. Temperature is constant and the slow reaction slightly increases degree of cure, accompanied by a small increase in ion viscosity (Rule 2 dominates). C. Temperature increases but is still too low for significant reaction or change in degree of cure, and ion viscosity decreases (Rule 1 dominates). D. At this higher constant temperature, the reaction and degree of cure increase significantly, causing a large increase in ion viscosity (Rule 2 dominates). E. Temperature decreases. Cure is complete by this time, degree of cure is constant and ion viscosity increases (Rule 1 dominates). Sometimes the changing temperature and cure rate create a more complex ion viscosity curve, as shown in Figure 21-2. Why does ion viscosity rise then fall between 90 and 150 minutes? Shouldn’t the reaction be accelerating because temperature is increasing during this time? Or is the cure somehow reversing?
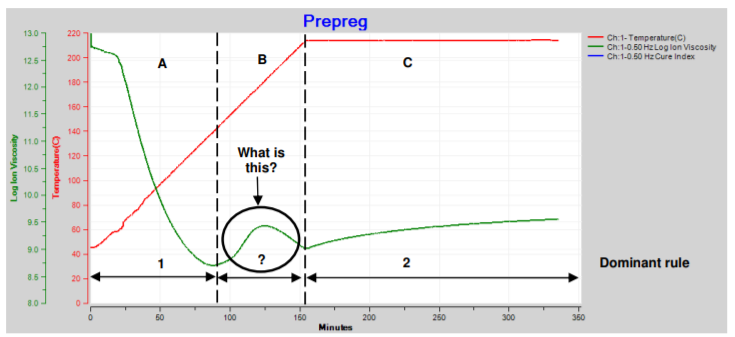
Epoxy-fiberglass prepreg cure with ramp and hold
Cure Index
For more insight, a parameter called Cure Index accounts for the effect of temperature and is closely related to degree of cure. Calculation of Cure Index requires two baselines that are characteristic of the material: one for the temperature dependence of ion viscosity at 0% cure and the second at 100% cure.
Any measurement of ion viscosity and the associated temperature now exists as a point between these baselines, as shown in Figure 21-3.
Cure Index is the vertical distance between this point and the 0% baseline, expressed as a proportion of the vertical distance between the two baselines.
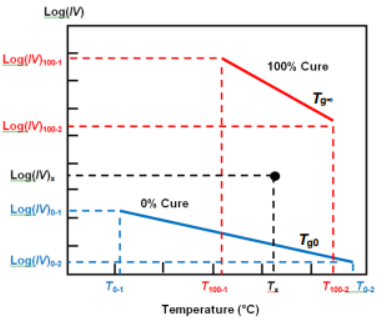
Cure Index baselines with ion viscosity-temperature data point
The 0% and 100% baselines are determined by measuring ion viscosity at different temperatures for uncured and fully cured material, respectively. Figure 21-4 shows the baselines obtained in this way for the prepreg of Figure 21-2. The ion viscosity-temperature data are plotted between them. The resulting Cure Index curve for this prepreg is shown in Figure 21-5 along with the original ion viscosity.
Reference :
Lambient TechnologiesTM Application Note 3.21—Measuring Degree of Cure With Dielectric Cure Monitoring, 1 Page
상세한 사항은 아래 첨부된 자료를 참고 부탁 드립니다.
